Are you Ready to Conduct a Study During COVID-19?
Help your team understand the FDA's recent guidance on conducting clinical trials during the pandemic.
Ensuring Clinical Trials Continue During the Pandemic
There are now more 750 clinical trials racing to deliver a medication or vaccine for COVID-19 and every clinical operations team is adapting to changing conditions on the fly. This pandemic represents a seismic shift in how clinical research is conducted and the FDA issued updated guidance on June 3rd to support the conduct of clinical trials during the health crisis, including the use of remote monitoring and digital tools.
As we are all finding ways to meet the challenge posed by the pandemic, the ArcheMedx team has accelerated development of a new learning and insights solution and free educational Activity, powered by Ready, to assist the clinical research industry in evaluating and improving understanding of the FDA’s new guidance on conducting clinical trials during COVID-19 .
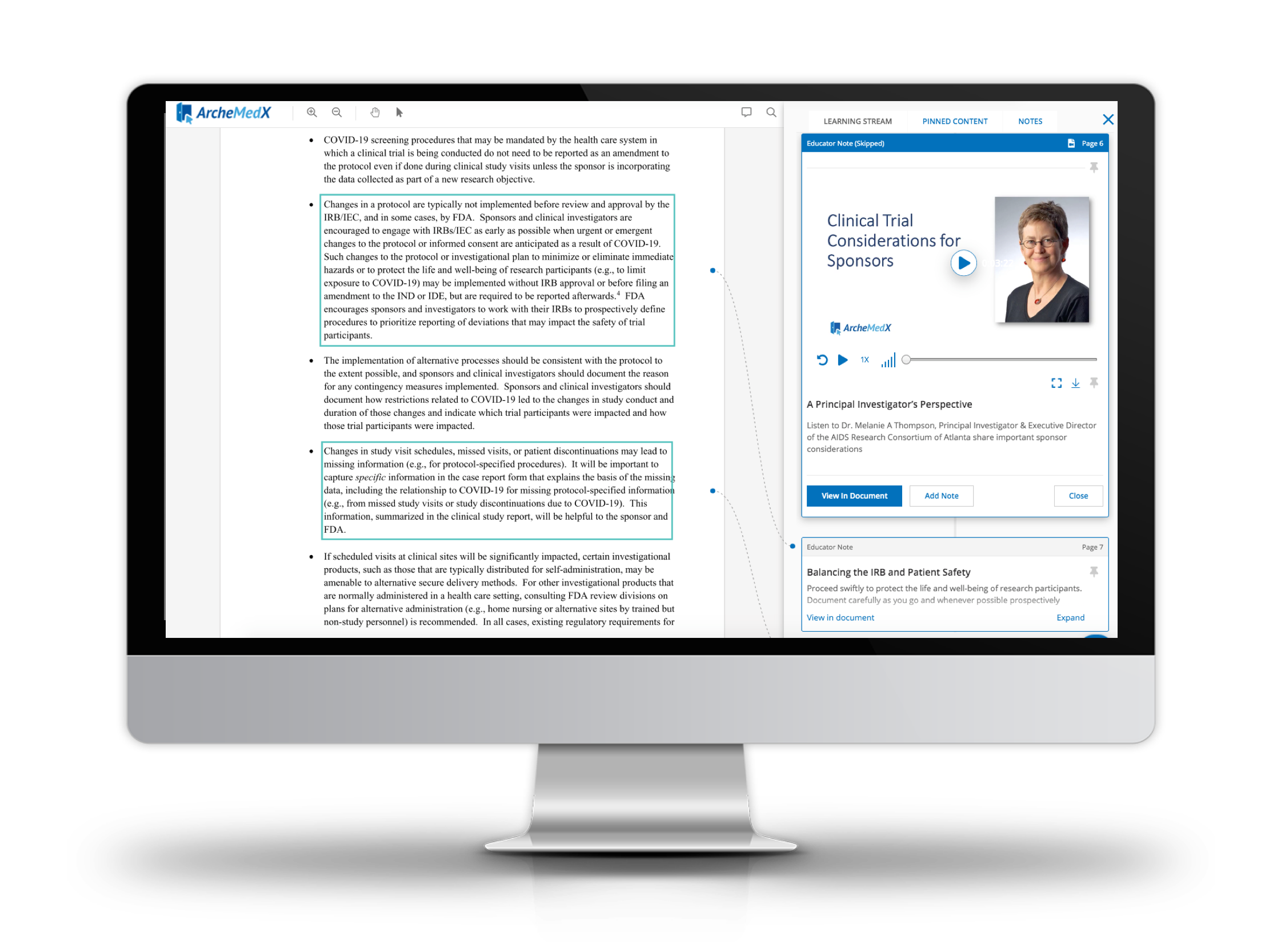
Understanding the FDA's Guidance on Conducting Studies During COVID-19
- This Activity is designed to improve your team's ability to conduct studies today by increasing understanding of the "FDA's Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic".
- It is made available free of charge to better equip clinical research professionals as they plan to conduct studies in this difficult environment and layers expert advice and practical tools for clinical operations professionals in and around the the FDA guideline
- It includes recommendations and resources curated by a highly experienced group of clinical research leaders, including Melanie Thompson, MD Principal Investigator & Executive Director of the AIDS Research Consortium of Atlanta and Amy Morris VP Clinical Development and Regulatory Affairs Trellis Bioscience.
A better way to learn
The FDA Activity is powered by Ready and the beta version of our new PDF centric learning and insights solution.
With Ready, you can rapidly transform any document into a personalized learning and assessment solution. Deliver a more engaging and individually tailored learning experience that layers multi-media resources and prescribed recommendations in and around your primary document as synchronized learning moments, making it easier for participants to discover, reflect, and dive deeper into critical information when it is most contextually relevant.
Ready also reduces traditional cognitive barriers to learning by enabling participants to:
- take digital notes synchronized to where they are in the content
- track learning moments and key resources for subsequent review and action
- search, view, and download a related library of resources and tools
- respond to personalized recommendations and engage in content boosts delivered over time to drive additional reflection and retention
Improving Our Broader Understanding
Since the ArcheMedX platform captures novel behavioral data and confidence measures as participants engage in the Activity, this data can be analyzed and shared (de-identified of course) with the broader community to indicate how the guidance is being understood across the industry and the likely actions clinical research leaders may take because of it.